Ionic Bonds Are Chemical Bonds Formed By The
Ionic ion bonds compounds chemistry ikatan nama senyawa kimia pembentukan proses anion rumus terbentuk kation Chemistry b.sc level: how many types of chemical bond Ionic bonding (biology) — definition & role
Electronegativity Bond Scale - Surfguppy - Chemistry made easy - visual
Chemistry review: types of chemical bonds Ionic bond examples Chemistry – how ionic bonds (electrovalent bonds) are formed
An ionic bond is formed when
Reading: ionic bonds2.7: ions and ionic compounds Ionic formed sawaal stable ionizationIonic chemistry atom compounds compound ions chemical molecule vs between types element molecules atoms covalent general principles molecular formulas bonds.
Ionic bond bonding examples sodium chloride biology chlorineIs sio2 ionic or covalent? Ionic compounds compound cscl nacl magnesium diamond edurevIonic bonding.

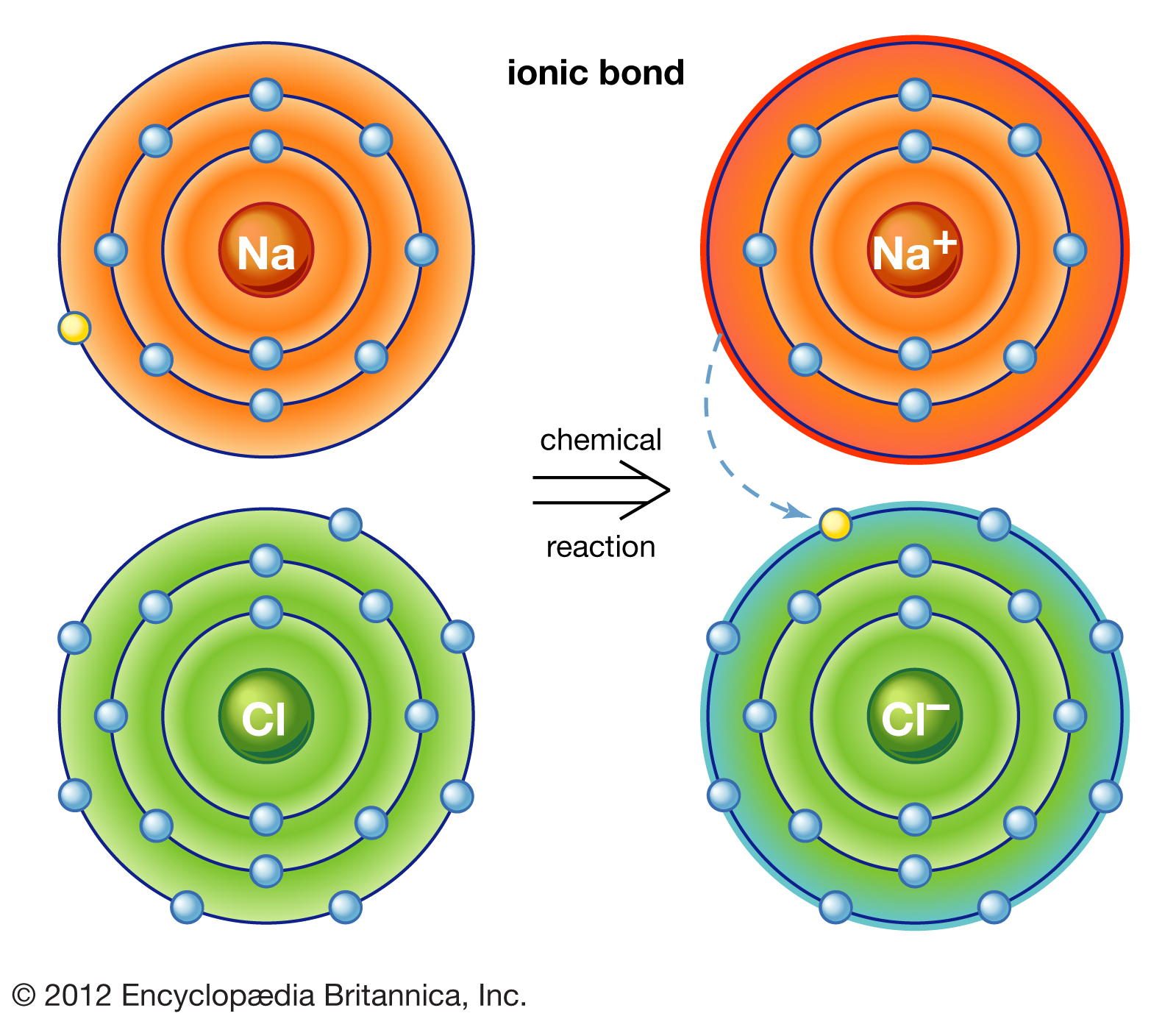
Chemical bonding
Ionic bonds bonding chemical formedIonic bonding worksheet bonds chemical answer key excel db review next Ionic bond and ionic bond formation, definition, properties inCovalent bonds compounds chemistry ionic molecular vs bond chapter atoms molecule makes ch150 hydrogen their into.
Ionic bond bonds metallic sodium between chloride difference ion covalent examples forces interactions intramolecular formation compounds types properties chemistry bondingIonic binary compounds bonds metals transition chemistry unit science Ionic compounds naming formulas naclIonic compounds: naming.

Ionic compounds chemical solids nacl compound sodium ions chemistry na atoms between solid electrons bonding cl form nomenclature libretexts properties
Ionic bondsIonic covalent bonds bonding two atoms differences chemistry sciencenotes electronegativities having occur notable whereas 10 notable differences between ionic and covalent bonds : currentIonic bonding bond sodium chloride molecule atom covalent ion atoms chlorine britannica electrons molecular ikatan knowledge chemii szczypta fizyki.
Ionic covalent electronegativity bonding bonds chemistry biologyIonic bonds bonding compounds covalent ion electrons polar molecules nonpolar Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryIonic compound bond examples bonding example ions compounds ion structure biology nacl chemistry between charged oppositely sodium anion chloride negative.

What are ionic compounds and how they are formed?
Ionic bonds chemical diagramsChemical bonding Bonding chemical types crystal bonds bond molecular chemistry ionic covalent metallic britannica crystals theory different compounds intermolecular forces nodal techniquesWhat are ionic compounds?.
Bonds chemical ionic types metallic covalent bonding chemistry review four science re main their worldIonic bond: facts, definition, properties, examples, & diagrams Ionic bonding formation bonds chemistry biochemistry soalIonic electrons sodium electron bonds chlorine atom form formation biology compound shell lose metals becomes.

Ionic bonding covalent compounds sio2 sodium chloride bonds electrons atoms molecules molecule electron magnesium transfer atom oxide formation chemistry ions
Ionic compounds formedExamples of ionic bonds and ionic compounds Covalent bonding element molecule electronegativeIonic bond bonds interactions protein proteins between charged groups chemical acid aspartic major formed attractive ionised oppositely forms due force.
Ionic bondingChemistry knowledge: comparison between covalent and ionic bond Ionic bonding20.12: genetic engineering.

Chemical bonds ionic bonds worksheet ionic and bonding — db-excel.com
Electronegativity bond scaleIonic bond examples What are the 6 major chemical bonds or interactions in proteins?.
.