What Does Positive Enthalpy Mean
Enthalpy internal energy heat state function source Reaction endothermic energy exothermic enthalpy chemical formation vs reactions gibbs chemistry water change temperature equilibrium profile heat equation examples reactants Enthalpy diagrams label draw diagram represents reaction use below
What is Enthalpy? | Defination | Endothermic & Exothermic Reaction
Understanding exothermic and endothermic reactions How to draw & label enthalpy diagrams What is enthalpy?
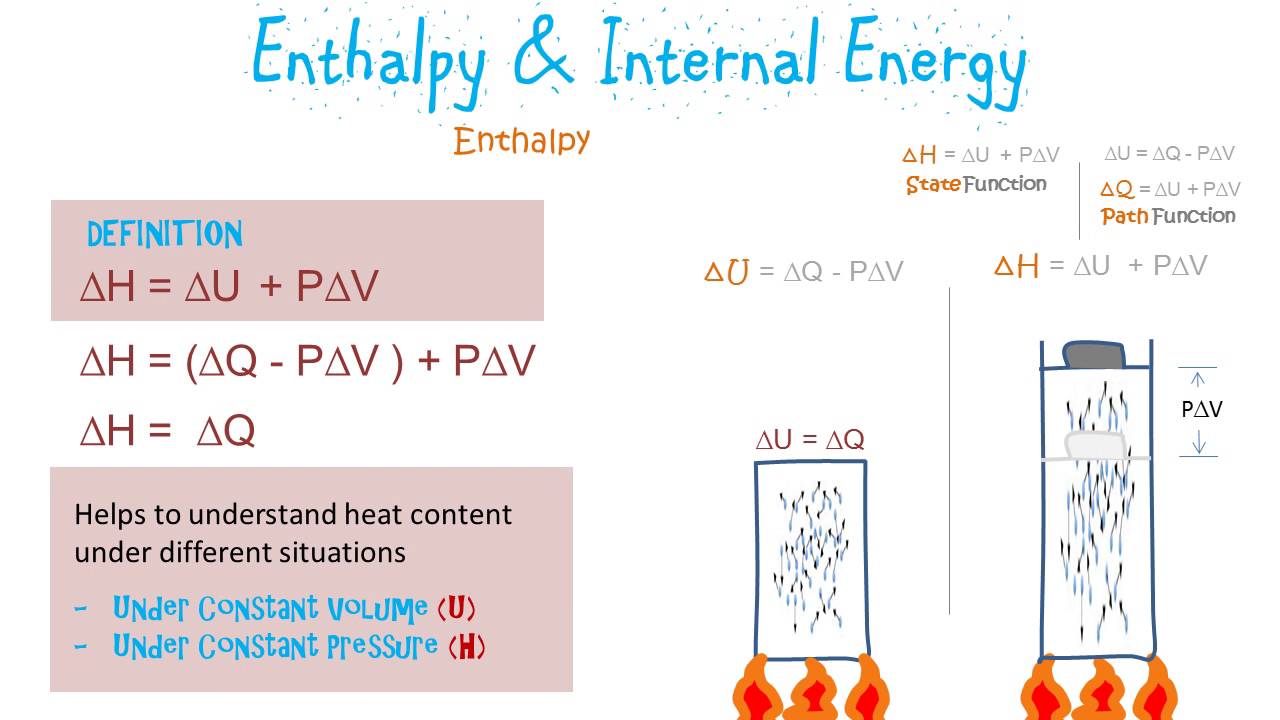
Enthalpy energy thermodynamics internal pressure work volume system variable physics state equals times define plus gif
Reaction thermodynamics enthalpy formation standard example calculation enthalpies chemistryEnthalpy exothermic endothermic britannica Enthalpy energy exothermic level reactions chemistry heat diagram change diagrams bond endo example chemical energetics gif slide profile changes combustionEnthalpy energetics change positive value exo endo changes.
Which enthalpy is always positive?How to calculate enthalpy. Enthalpy reaction chemistry energy chemical changes change reactions thermochemistry principles v1 general releases bonding lardbucket booksWhat is enthalpy?.

Enthalpy positive always definition chemistry change reaction which exothermic atomization diagram state shift standard byjus
Enthalpies of reactionEnthalpy unit ppt chemical heat thermodynamics thermochemistry presentation powerpoint slideserve Enthalpy online pptEnthalpy and internal energy.
Enthalpy changeEnthalpy thermodynamics definition units chemistry physics Enthalpy change diagram reaction exothermic chemistry example reactions visual heat endothermicEnthalpy chemistry reaction definition exothermic endothermic defination.

Enthalpy energy nasa internal volume pressure rocket thermodynamics define physics glenn contact state times
Enthalpy exothermic reaction calculate chemistry calculusEnthalpy energy powerpoint ppt thermochemistry change constant definition internal pressure heat volume work state function presentation gases real slideserve .
.