What Forms When Two Atoms Share Electrons
Solved please answer them all, this is my last Biological chemistry organic general basics electrons 1. electron configuration
Electron Energy Levels of Atoms | Image License | Carlson Stock Art
Chemical reactions and molecules Electrons energy levels electron atom nucleus around arrangement shell shells atoms subshells sublevels configuration chemistry main level maximum atomic structure Edumission: chemistry form 4: chapter 2
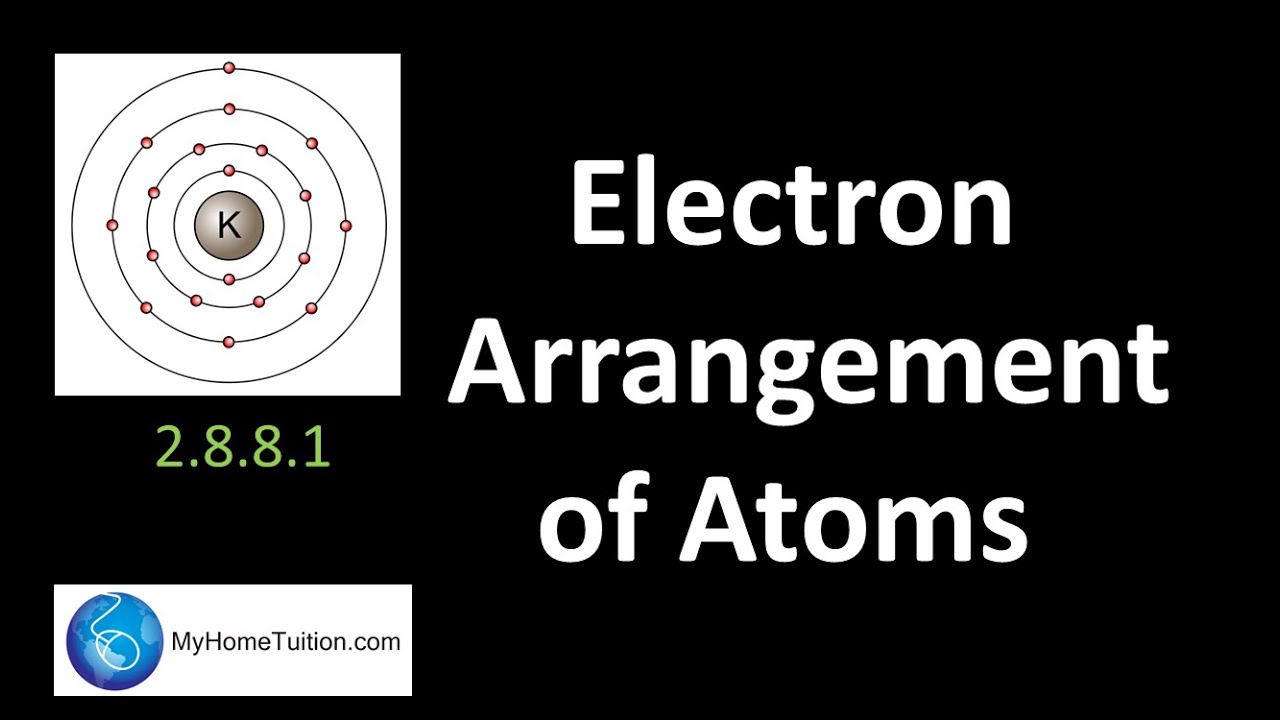
Susunan elektron di dalam atom
Ionic bondsExamples question atoms electrons between shared example these Bonding ikatan kovalen lewis covalent kimia atoms bonds atom bagaimana bergabung theory libretexts molecules form sainskimia electronsQuestion #49ca9 + example.
Atoms sharing electron bonding electrons bond covalent two when formed chapter chemical ppt powerpoint presentation slideserveWhat does it mean if atoms have the same atomic number but a different Atoms bond molecules form two each oxygen molecule other electrons when hydrogens forms water hydrogen covalent chemical atom electron reactionChemical bonds · anatomy and physiology.

Periodic table compounds chemistry ionic bonds covalent valence each ions element elements electron family lewis molecular symbols has dot ch150
Atoms, isotopes, ions, and molecules: the building blocksOxygen atom – chuba oyolu's portfolio Atom electron spmCh150: chapter 4 – covalent bonds and molecular compounds – chemistry.
Lewis theory of bondingChlorine cl2 molecule covalent atoms electrons socratic Energy electron levels atoms structure molecularElectron atom nucleus configuration electrons number energy atomic levels protons each orbit mass neutrons.

Atoms atomic number neutron proton atom electron mean same different does if but mass chemistry socratic model questions begin definitions
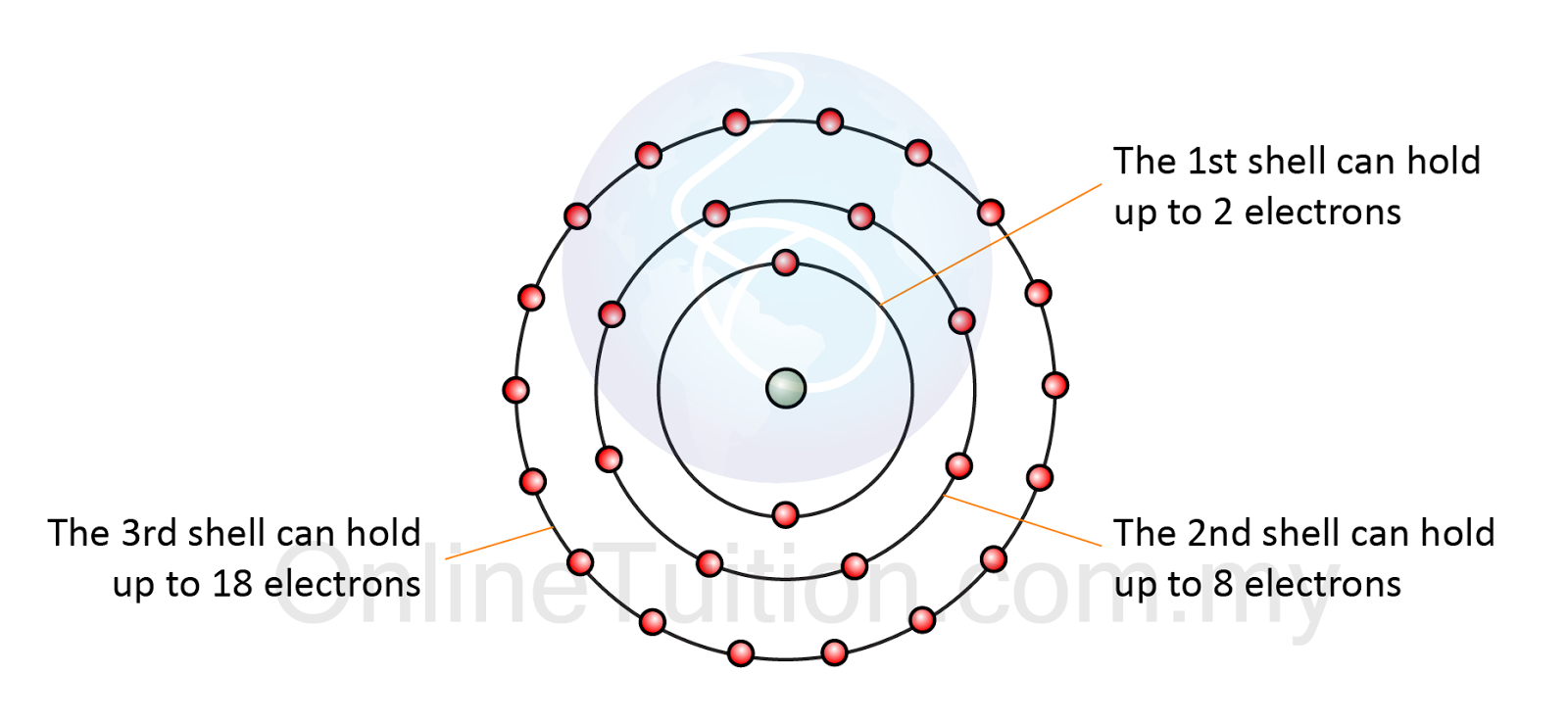
Covalent electrons atoms pairs bonds dots2.6 arrangements of electrons Electron energy levels of atomsElektron susunan kimia dalam tingkatan nota shell padat bohr dapatkan berguna.
Atoms hydrogen electrons two covalent molecule form bonds shared bond hillis2e combine electron figure ch02Oxygen molecular atoms molecules between atomic hydrogen chemical o2 bond molecule difference bohr model double reactions biology formation ions electrons Electron arrangement in atomHillis2e_ch02.

Electron configuration orbitals electrons orbit notation space pairs
Chlorine combined with two negative atom or 1 positive and other2.1 – atoms, isotopes, ions, and molecules: the building blocks Bonds hydrogen molecule water chemical anatomy bond covalent structure oxygen polar atoms atom negative electrons two model structural three endIonic bonds bond ions atom example nacl na ion electrons cl bonding electron atoms valence gain chemistry lose edu geo.
Atoms electrons ionic compounds covalent nacl bondsOxygen atom electrons ring outermost Atoms oxygen molecules ions isotopes valence molecule blocks electrons psu unpaired cuny paired1. electron configuration.