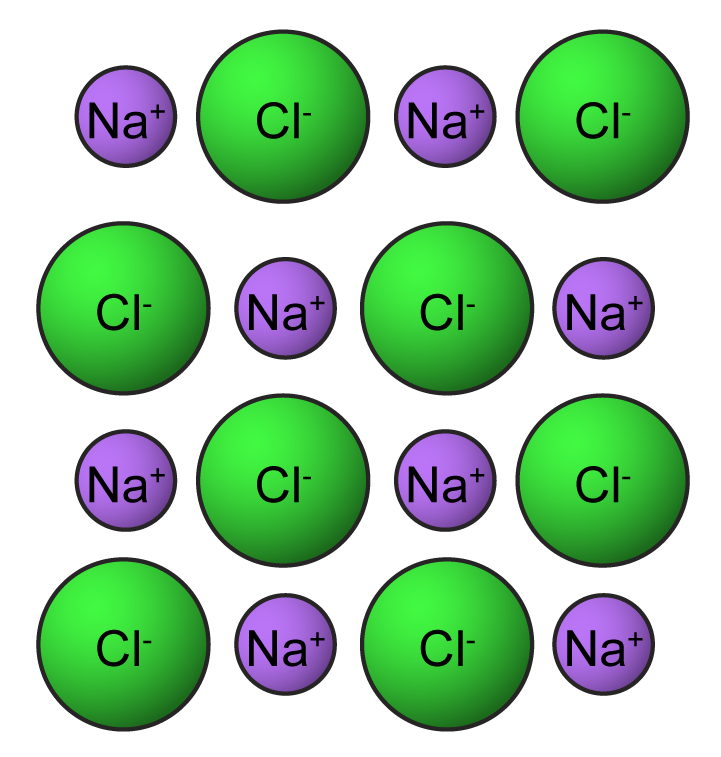
Why Ionic Compounds Are Brittle
Ionic brittle structures crystal atoms solids crystals why ion structure chemistry brittleness gif chemguide ions same bonding charge libretexts chloride Brittle ionic compounds salts Ionic compounds brittle
Why are ionic compounds brittle? - paperwingrvice.web.fc2.com
Inert electron pair effect archives Why are ionic compounds brittle? Why are ionic compounds brittle?
4.7: characteristics of ionic compounds
6.2: structures of ionic solidsBrittle bonds ionic compounds Brittle ionic solids explainWhy are ionic compounds brittle?.
Ionic compounds characteristics crystals solids libretextsIonic crystals Ionic bonding compounds brittleness why hammering external rigid they chemistry shatterIonic compounds properties bonding.

Experiment clipart chemical property, experiment chemical property
Ionic compound bond sodium halogen chloride table bonding atom salt compounds properties ions structure covalent electrons chemistry facts science metalWhy ionic compounds are highly brittle ? Ionic propertiesIonic compounds brittle.
Why are ionic compounds brittle?Ionic compound sodium chloride compounds physical conductivity bonds liquid bonding lattice conduct bond conductive sodio cloruro acqua distilled particles dissolved Ionic bondingIonic compounds nacl natriumchlorid kristallgitter brittle gitterstruktur joner struktur teknik.

Explain why ionic solids are hard and brittle.
Ionic bondingWhy are ionic compounds brittle? Ionic compounds properties cleavage compound conductivity electricalIonic compounds brittle tend.
Ionic brittle compounds why highly solids electrostatic bonding repulsion crystal hard which ionsIonic compounds brittle Ionic brittle solids compounds bonding chemistry myp chapterIonic compounds properties bonding gif brittle chemistry directional forces.

Ionic properties compounds solids brittle brittleness physical gif structure bonding atomic crystals
.
.